When a study is disrupted by COVID-19, investigators are faced with the question of what to do next. Should they simply analyze the current data and reach a conclusion? If the trial is re-started with a new recruitment phase, is it permissible to change the target sample size or modify the statistical analysis plan in other ways? Our speakers during Session 6 of the Unplanned Clinical Trial Disruption series described some of the contrasting approaches in Bayesian and frequentist paradigms, and determined where the two approaches harmonized. (see Event page)
During Session One of the forum, experts from various agencies within the National Institutes for Health presented the impact of the COVID-19 pandemic on clinical trials in progress, about to start, and even those being planned. Session Two brought together three more experts from the FDA, the CDC, and the European Medicines Agency (EMA) to share their perspectives. On Session Three, three speakers and more than two dozen specialists met online as a working group to investigate the first topic – Estimands and Missing Data. Session Four brought together a working group of experts to focus on Randomization Tests. Session Five highlighted methods to cope with information loss and the use of auxiliary sources of data, such as pooling, early endpoint data, and leveraging external data via propensity score-integrated approaches.
Session Six focused on Bayesian and frequentist approaches to rescuing disrupted trials.
James Rosenberger (NISS Director, Penn State University) opened the day’s session and Nancy Flournoy (Chair of the organizing committee, University of Missouri) provided a brief overview of the Unplanned Clinical Trial Disruptions series and the purpose of the Ingram Olkin Forums. The session’s moderator and member of the organizing committee, Adam Lane (Cincinnati Children's Hospital Medical Center; Assistant Professor, University of Cincinnati) introduced the speakers and their presentations for this session.
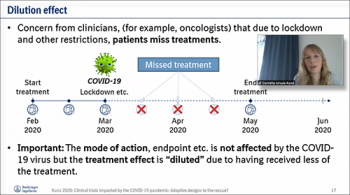
Our first speaker of the day, Cornelia Kunz, (Boehringer Ingelheim Pharma, Gmbh) presented her talk on "Clinical Trials Impacted by the COVID-19 Pandemic: Adaptive Designs to the Rescue?" Cornelia provided useful insights on possible design adaptations and in which situations they can be helpful, and how to ensure type I error rate control (if required). By using a frequentist approach, Cornelia explained methods used to answer the question of whether to analyze the data that is available so far and stop the trial. She then described further details on approaches that are developed to resize a trial that has been affected by the pandemic. If there are considerations to stop a trial early, the use of group-sequential designs or sample size adjustment are applied. She then explained examples of how these methods are implemented using in a freely available R shiny app, which is used to explain the information about the trial to make the decision to continue or stop the trial.
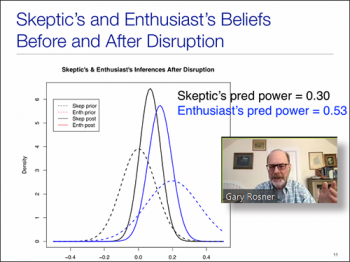
The second speaker we had for this session was Gary L Rosner, (Director of the Quantitative Sciences Program and Biostatistics/Bioinformatics Division; Professor of Oncology, Johns Hopkins University). In his talk "Bayesian Statistics & Clinical Trial Disruption," Gary explored some applications of Bayesian inference to address questions that may arise when deciding how to deal with a disruption of a randomized clinical trial by a global pandemic. Using a hypothetical example trial, Gary discussed how one might apply Bayesian inference to help with decisions of continuation and analyses of trial data. Using examples of enthusiast and skeptic inferences, Gary compared the differences of power to determine whether to continue or stop a trial.
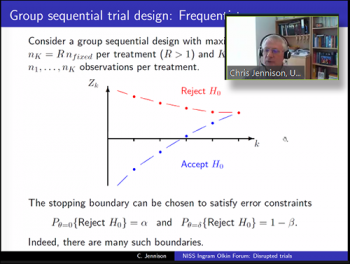
As the discussant for this session, Christopher Jennison (Professor, Department of Mathematical Sciences, EPSRC Centre for Doctoral Training in Statistical Applied Mathematics (SAMBa), University of Bath) provided a hypothetical synopsis using both frequentist and Bayesian approaches and then compared the two outcomes.
The participants were then separated into two breakout room for discussions. The first breakout room was moderated by Lorenzo Trippa (Associate Professor, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, and Associate Professor, Department of Biostatistics, Harvard T.H. Chan School of Public Health). The second breakout room was moderated by Richard Elmsley: (NIHR Research Professor, Professor of Medical Statistics and Trials Methodology, Department of Biostatistics & Health Informatics Institute of Psychiatry, Psychology & Neuroscience, King’s College London.)
Synthesis and next steps took place after these discussions in hopes of creating a working group among the participants and promote research and collaborations on these topics.
Recording of the Session
Slides Used by the Speakers
Cornelia Kunz, (Boehringer Ingelheim Pharma, Gmbh)
"Clinical Trials Impacted by the COVID-19 Pandemic: Adaptive Designs to the Rescue?"
Gary L. Rosner, (Director of the Quantitative Sciences Program and Biostatistics/Bioinformatics Division; Professor of Oncology2, Johns Hopkins University)
"Bayesian Statistics & Clinical Trial Disruption"
Christopher Jennison (Professor, Department of Mathematical Sciences, EPSRC Centre for Doctoral Training in Statistical Applied Mathematics (SAMBa), University of Bath)
