
This session involved members of the Student and Young Professionals Outreach Committee of the Non-clinical Biostatisticians Working Group (NCBWG), BioPharmaceutical Section of the American Statistical Association (ASA). They included Tom Bradstreet (Director at Bristol-Myers Squibb), Oluyemi Oyeniran (Principal Statistician at Johnson & Johnson), Jyh-Ming Shoung (Director at Johnson & Johnson), and Fanni Zhang (Senior Statistician at AstraZeneca), who served as moderator of this event. Wei Zhao of Fate Therapeutics was invited but had a schedule conflict. (see Event page)
NISS has once again gathered an incredible set of speakers/panelists. Not only did they bring extensive experience working in the field of biostatistics, but these individuals have as their priority a willingness to share their experiences so that others may better understand and be better prepared for working as a non-clinical biostatistician.
Tom Bradstreet (Bristol-Myers Squibb) introduced the session explaining their purpose was to introduce the drug research and development process, how non-clinical statisticians are involved in each step, to provide attendees with a better understanding of different career paths in statistics that exist in the pharmaceutical industry.
So, what are the challenges that are unique to biostatisticians in non-clinical settings? What types of opportunities are available? How should one prepare to enter this field?
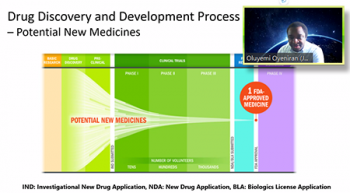
Oluyemi Oyeniran (Johnson & Johnson) provided a thorough presentation that involved three parts: an overview of drug discovery and drug development process, an overview of non-cinical statistics, and a look at non-clinical statisticians in the pharmaceutical industry. Oluyemi clearly outlined the discovery and development process from early drug discovery through to the FDA review and post-market monitoring. On top of this, he laid out the types of work that non-clinical biostatisticians get involved in. He pointed out: data science; preclinical drug discovery; safety and toxicology; and chemistry, manufacturing and control (CM&C) processes. From here Oluyemi talked about the skills that non-clinical biostatisticians will need in order to do this work. He stressed not only the importance of being able to use statistical tools such as linear and nonlinear models, repeated measures, statistical process control, multivariate analysis, Bayesian strategies, among others, but also the importance of being able to communicate and work well with other scientists.
“Without data you’re just another person with an opinion.”
Oluyemi quotes the sage advice of W. Edwards Deming
A final emphasis of Oluyemi’s presentation was the urging of attendees to get involved in the NCB literature and attend the currently bi-annual ASA Biopharmaceutical Section Nonclinical Biostatistics Conference. This year the conference is being held June 21-24, 2021 and will be virtual.
Moderator Fanni Zhang (AstraZeneca) fielded questions from attendees which included: “Are publications possible when working as a non-clinical statistician?”, “Do you have suggestions for early-career statisticians/data scientists of what types of projects to include in their portfolio?” and “Are there any job openings at your institution now and what are your expectations?”
If you are thinking of seeking a position in the pharmaceutical industry, then this is certainly a session that you are going to want to review! Play the recording of this session along with the copies of the slides that the speakers used below. The slides not only provide you with the key points that were offered, but also include links to additional resources and contacts that should not be ignored!
Recording of the Session
Slides used by the Speaker
Oluyemi Oyeniran (Principal Statistician at Johnson & Johnson
