


[Please Note: The program for this Ingram Olkin Forum on Unplanned Clinical Trial Disruptions is available at: Session Three.
September 1, 2020
What are the implications of unplanned disruptions of clinical trials? What strategies can be put into place to ensure the integrity of statistics using data that have already or will not be collected? Are there changes to methods or plans that researchers can put into place moving forward?
Day Three was the first of a series of smaller working groups planned to investigate topics related to methods or strategies for mitigating disruptions.
Previous to this working group, on Day One of the forum three experts from various agencies within the National Institutes for Health spoke concerning the impact of the COVID-19 pandemic on clinical trials in progress, about to start and even those being planned. They included Nancy L. Geller, the Director of the Office of Biostatistics Research at the NHLBI, Dean Follmann, Chief at the Biostatistics Research Branch at the NIAID and Lisa McShane, an Associate Director at the Division of Cancer Treatment & Diagnosis and Chief of the Biometric Research Program at the NCI. Day Two brought together Lilly Yue, Deputy Director, Division of Biostatistics, Center for Devices and Radiological Health at the FDA, G. David Williamson, Director, Office of Science, National Center for Environmental Health at the CDC and Aldana Rosso, a representative of the European Medicines Agency, EMA to share their perspectives.
These first two days of open presentations helped set the stage for further investigation. The organizing committee sent a questionnaire to all participants soliciting interest for active participation on Days 3-7 in small discussion groups in breakout rooms, regrouping for reporting, syntheses, and writing on the topic of the day,
On Day 3, more than two dozen specialists with specific interest met online as a working group to investigate the first topic – estimands and missing data. In recent years there has been increased scrutiny about precisely what treatment effect is being estimated (the estimand) by a clinical trial, in the context of complications such as treatment switching and discontinuation and competing risks.
Three speakers again directed their comments to this specific topic. David Murray, (Associate Director for Disease Prevention and Director of the Office of Disease Prevention (ODP)) provided an overview of the design and analytic methods for traditional randomized controlled trials and focused on the designs that involve randomization of groups or clusters or delivery of interventions in a group-format. He used examples from the Health Care Systems Collaboratory Project and reviewed how the trials adapted in response to various disruptions. In conclusion, David highlighted the importance of considering the elements of population, intervention, comparator, outcome, and time frame (PICOTs) as well as considering the CONSORT Guidelines.
The second speaker was Mouna Akacha, the Group Head of the Statistical Methodology group of Novartis Pharma AG, based in Basel, Switzerland. Mouna discussed the value of the estimand framework (ICH 2019) in assessing the impact of the pandemic on the scientific question of interest targeted by a given trial, identifying the relevant data and information related to the pandemic that needs to be collected, and being able to support the proper analysis and interpretation of the trial data. After describing a number of examples, Mouna concluded that the estimand framework helps to structure the problem by assessing the impact of complications on the trial integrity and also helps to distinguish between intercurrent events and missing data. However, she acknowledged that alignment is needed regarding the choice of estimand with respect to the unforeseen intercurrent events and that robust estimation may be challenging.
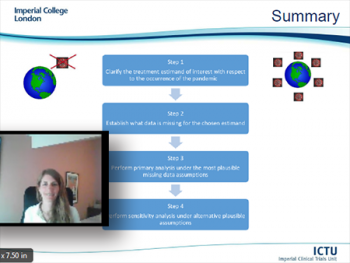
The final speaker of Day Three was Suzie Cro, Research Fellow at Imperial Clinical Trials Unit (ICTU), School of Public Health, Imperial College of London. Suzie explored a four-step strategy for handling unplanned disruptions in the analysis of randomized trials that are ongoing during a pandemic using missing data methods. Based on a framework consistent with the statistical principles outlined in the ICH-E9(R1) addendum on estimands and sensitivity analysis in clinical trials, Suzie’s talk described each point of the guidance in turn through an example from an ophthalmic trial ongoing during COVID-19. She ended her presentation with a review of questions related to choosing the most appropriate treatment estimand with respect to the pandemic, identifying affected data and the application of non-missing data methods to target these estimands as well as considering alternative modeling methods.
Right after these informative and insightful talks, all of the meeting participants were ushered into two breakout rooms. One room was given the task to identify and formulate open statistical methodological questions related to COVID-19 driven disruptions. A second breakout room was tasked with considering applied problems related to COVID-19 driven disruptions. These two groups were moderated by Toshimitsu Hamasaki (George Washington University) and Frank Bretz (Novartis) who reported back to the forum at the conclusion of the event. Being a small group a brief round of general questions and discussion of next steps followed. A small working group will be organized for the purpose of synthesizing recommendations based on these discussions.
Days 5 and 6 on aspects of adaptive designs in the context of unplanned disruptions are in the planning stage. The organizing committee is aiming for October 20, 2020 for Day 4. So, hold this date (10 am -1 pm ET) and watch for future announcements!
Recording of the Speakers
Slides used by the Speakers
David Murray, (Office of Disease Prevention, NIH)
Mouna Akacha, (Novartis)
"COVID-19 – Estimands to the rescue?"
Suzie M. Cro, (Imperial College of London)
"Handling unplanned disruptions in randomized trials using missing data methods: a four-step strategy"
