[Please Note: The program for this Ingram Olkin Forum on Unplanned Clinical Trial Disruptions is available at: Session Five.
In some studies, disrupted by COVID-19, continued follow-up of existing patients and recruitment of new patients has not been possible, leading to smaller sample sizes and less complete information than had been planned. This session’s speakers explored methods and explained the approaches to overcome such information shortfalls. One approach uses short term endpoints to replace or augment the original endpoint. Another uses historical data and/or concurrent data from external sources. During Session Five of the Unplanned Clinical Trial Disruption series, the speakers described such approaches and outlined the challenges they pose.
During Session One of the forum, experts from various agencies within the National Institutes for Health presented the impact of the COVID-19 pandemic on clinical trials in progress, about to start, and even those being planned. Session Two brought together three more experts from the FDA, the CDC, and the European Medicines Agency (EMA) to share their perspectives. On Session Three, three speakers and more than two dozen specialists met online as a working group to investigate the first topic – Estimands and Missing Data. Session Four brought together a working group of experts to focus on Randomization Tests.
Session Five of this series focused on coping with information loss and the use of auxiliary sources of data.
James Rosenberger (NISS Director, Penn State University) opened the day’s session and Nancy Flournoy (Chair of the organizing committee, University of Missouri) provided a brief overview of the Unplanned Clinical Trial Disruptions series and the purpose of the Ingram Olkin Forums. The session’s moderator Chris Jennison (Moderator, University of Bath) introduced the speakers and their presentations for this session.
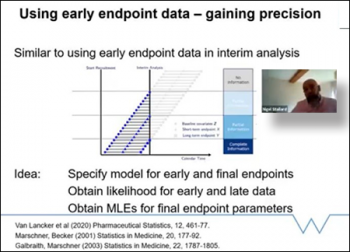
The first speaker was Nigel Stallard, (Warwick Medical School). Nigel began by applauding the huge effort that has gone into clinical research, and the thousands of trials which have led to enormous advances in treatment and vaccines. Due to COVID-19 many ongoing trials have been disrupted, especially those labeled as non-essential. Although many will start again and continue, some will not be able to. If early endpoint data is available however, how can we use that data to learn from the information we have available? Can we somehow use information from earlier outcomes to help us in our inference on the primary endpoint? Nigel provides the answer: “Yes we can!” We can use early endpoint data to help us learn about the primary outcome and incomplete data can be analyzed at interim analysis. Nigel continues his talk going through examples on how we can make that work through binary data, normal data, and gaining precision models on the estimation of death.
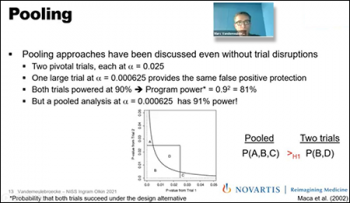
Our second speaker for the day, Marc Vandemeulebroecke, (Novartis), identifies ways that leveraging external data could help mitigate the impact of “force majeure” trial disruptions. The term “force majeure” pertains to the unexpected event that prevents the continuation of work in clinical trials. Marc continues to explain approaches to compensate power loss when the trial is disrupted. While various types of data sources could be considered such as historical data, concurrent data, real-world data, etc. there are different types of techniques that could be used to incorporate information into a trial. Marc highlights pooling analysis as a quantitative approach being very beneficial to accommodate for loss of power in a clinical trial. After conducting a pooling analysis in a case study example using real-world data, Marc shows that the pooled analysis at those trials have a much greater power compared to the individual approaches in the example.
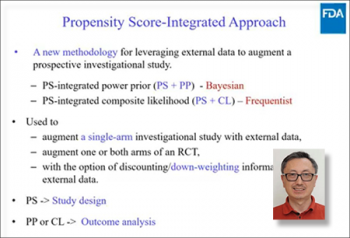
The final speaker of the day Heng Li, (Division of Biostatistics, FDA, NIH), highlighted leveraging external data via the propensity score-integrated approach. Examples of practical procedures were provided during this session on salvaging those stopped studies for which restarting enrollment is not feasible, by integrating external patients with data already collected to recover the loss of study power due to premature stopping. The propensity score-integrated approach includes prior power (Bayesian) and composite likelihood (frequentist) procedures. Heng Li proceeded with further examples of both approaches to mitigate information loss.
Breakout discussion groups following the presentations in Zoom were led by Lisa Hampson, (Moderator, Novartis, Switzerland) and Sarah Zohar (Moderator, INSERM, France). The purpose of these breakout group discussions was to identify questions needing further research and develop collaborations between the statisticians and practitioners to investigate these methods further in order to implement them in practice. Synthesis and planning next steps were discussed upon closing the day’s session.
What’s Up Next?
There will be a Session Six session in the series Unplanned Clinical Trial Disruptions that will be held on April 27, 2021 on Bayesian and Frequentist approaches to rescuing disrupted trials! Event details will be posted soon!
Recording of the Session
Slides Used by the Speakers
Nigel Stallard, (Warwick Medical School)
"Using short term endpoint data in interrupted clinical trials"
Marc Vandemeulebroecke, (Novartis)
Heng Li, (FDA, NIH)
